ISSN: 2688-562X
Research Article
Diagnostic and Prognostic Value of Monocarboxylate Transporters Gene Expression in Patients with Prostate Cancer and Its Potential Use in Liquid Biopsies: A Preliminary Study
Matheus Moreira Perez1, Glaucia Luciano da Veiga1, Alexandre Luiz Affonso Fonseca1, Luiz Vinícius de Alcântara Sousa2, Auro del Giglio3, Fernando Luiz Affonso Fonseca1,4, and Beatriz da Costa Aguiar Alves1*
- 1Clinical Analysis Laboratory, ABC Medical School (FMABC), Brazil
- 2Laboratory of Epidemiology and Data Analysis, ABC Medical School (FMABC), Brazil
- 3Discipline of Oncology and Hematology, ABC School of Medicine (FMABC), Brazil
- 4Institute of Pharmaceutical Sciences, Federal University of São Paulo (UNIFESP), Brazil
- *Corresponding author: Beatriz da Costa Aguiar Alves, Clinical Analysis Laboratory of the ABC School of Medicine, Lauro Gomes, 2000, ZIP Code: 09060-870 Santo André, SP, Brazil, Email: bcaalves@uol.com.br
- Received: Aug 13, 2019 Accepted: Aug 27, 2019 Published: Aug 29, 2019
Abstract
Prostate cancer (PCa) is the second most common type of tumor in men and the need to establish new biomarkers that may aid those already existing in the diagnosis and follow-up of the disease is becoming increasingly evident. In order to maintain the intracellular pH, the cancer cell regulates the production of monocarboxylate transporter proteins (MCTs). We aimed to evaluate the potential prognostic and diagnostic value of MCT1, MCT4 and CD147 gene expression in the blood of patients with PCa throughout the treatment. The difference in expression of the proposed markers between patients with PCa (n = 27) and healthy men (n = 26) was obtained by RT-qPCR and determined by the 2-ΔCq method. Patients with PCa and healthy donors differ in the expression of MCT1, MCT4 and CD147, and this difference can be detected in peripheral blood samples. MCT1 presented higher expression in patients with PCa than in healthy subjects, progressing in line with the severity of the disease. In the analysis of the ROC curve, MCT1 expression showed a high specificity. CD147 area under the curve, although lower than that obtained for MCT1, is simila0r to that described for % free PSA (0,64) and higher than that described for free PSA (0,615). Detection of MCT1 expression in peripheral blood samples could be a complementary diagnostic tool as well as a prognosis marker in prostate cancer.
Keywords:
Prostate cancer; MCT1; Differential gene expression; Diagnosis; Prognosis
Introduction
Prostate cancer (PCa) is the second most common type of tumor among men, with the first being non-melanoma skin tumors. According to the National Cancer Institute (INCA), there were an estimated 68,220 new cases of prostate cancer in Brazil in 2018 [1]. Age and race are the main risk factors for the development of the disease, which affects mainly black men and those over 65 years old [2]. Although most men have the disease with a low to intermediate risk of metastatic onset, 15% of them have the disease at an advanced stage. The incidence of metastatic disease in patients or the high risk of developing metastases has increased [3].
The diagnosis of PCa is mainly performed by transrectal guided ultrasound biopsy, a technique that has been replaced by less invasive imaging tests, such as multiparametric magnetic resonance imaging and positron emission computed tomography (PET-CT) [4]. In addition to imaging tests, digital rectal examination and laboratory parameters help in the early detection of the disease and follow-up.
Currently, the prostatic specific antigen (PSA) is the most used laboratory parameter to complement the diagnosis and follow-up of PCa [5]. PSA, a glycoprotein produced by the prostate gland that helps sperm motility and dissolution of the cervical mucus, is elevated in patients with PCa, benign tumors, and other prostatic comorbidities [6].
Histological classification of PCa is most commonly performed based on the Gleason classification system. This method is based on structural features of cancer cells. The more undifferentiated and anaplastic the affected tissue, the greater the Gleason score, which ranges from 2-10. Along with stage, age and PSA levels, the Gleason score is a predictor of prognostic outcome, a determinant factor in therapeutic decision making. For this reason, the Gleason score is used as a parameter to verify the association of markers with the stage and prognosis of PCa [7].
Because changes in PSA values are not specific to PCa, other diagnostic and prognostic biomarkers have been studied [6]. Among them, the Human Epidermal Growth Factor Receptor type 2 (HER2) is mentioned, and its increase in serum concentration can increase rates of cellular transformation, affecting apoptosis-related pathways, among others [8].
More specific methods of detection and prognosis of PCa have been developed, especially those based on analysis of gene expression by real-time polymerase chain reaction (qPCR) and its variants. An example of a biomarker evaluated by this method is the Androgen Receptor variant 7 (ARv7), associated with castration-resistant PCa [9]. Recently, liquid biopsies have been attracting interest, since it is a noninvasive technique that evaluates these biomarkers, helping to monitor the disease. Blood is the most widely used material because it contains Circulating Tumor Cells (CTCs), and detection of these is associated with a reduction of life expectancy in several types of cancer [10-12].
The main features common to the different types of malignancy are evasion to apoptosis and growth inhibitory factors, the ability to promote angiogenesis and self-sustaining proliferative signaling, which may acquire invasion capacity and metastasis [13]. All these characteristics, especially the rapid cellular proliferation, result in malignant cells being highly glycolytic due to their great energetic demand. Although aerobic energy production with oxidative phosphorylation (Oxphos) is more efficient than anaerobic, the tumor microenvironment is characterized by an exacerbated rate of energy production that occurs preferentially via anaerobic glycolysis, even in the presence of oxygen, leading to high production of acids, especially lactate. This phenomenon is known as the “Warburg effect” [14,15]. This leads to an acidic tumor environment, which is associated with an increase in several tumor characteristics such as cell migration, invasion and metastasis. To prevent cell death caused by acidosis, tumor cells increase proton efflux in order to maintain intracellular pH. This maintenance of pH is accomplished through the regulation of proton pumps, bicarbonate carriers and monocarboxylate carriers (MCTs).
MCTs are part of the SLC superfamily of solute carriers, comprising 14 isoforms [16,17] that facilitate the transmembrane transport of the end products of glycolysis and short chain fatty acids, associated with a proton, such as pyruvate and L-lactate. L-lactate is important both in glycolytic/oxidative metabolism and in the signaling of the promotion of angiogenesis and immunosuppression [18]. Among the members of this family, only MCTs 1-4 have the ability to transport monocarboxylates coupled to a proton through the cell membrane [19-21]. Some of the MCTs isoforms are overexpressed in several types of cancer, playing a central role in stromal-parenchymal tumors and endothelial cell metabolism and, consequently, they have been shown to be potential therapeutic targets in the treatment of cancer [22].
MCT1 (SLC16A1) gene is expressed in most of the tissues studied and is involved in both the uptake and efflux of monocarboxylates, being more commonly found in normoxic cells. MCT4 (SLC16A3) gene expression is largely restricted to tissues that perform anaerobic glycolysis, in which, despite having less affinity for L-lactate than MCT1, is involved with its efflux [23,24]. MCT1 and MCT4 genes are regulated by an association with the glycoprotein cluster of differentiation 147 (CD147). CD147 functions as a chaperone responsible for cytoplasmic trafficking and anchorage of these and other membrane proteins, angiogenesis and extracellular matrix modeling [25]. Overexpression of this glycoprotein is observed in many malignancies and is correlated with pathological functions that promote tumor progression, such as proliferation and angiogenesis [26].
Previous work of our group, also using liquid biopsies, showed relationships between the gene expressions of MCT1 and MCT4 monocarboxylate transporters and the chaperone CD147 in the blood of patients with breast cancer receiving chemotherapy.
MCT1 and CD147 expression were potential diagnostic markers, and MCT4 proved to be a potential prognostic marker [27]. Due to the dearth of research relating the expression of these markers in liquid biopsies, the objective of this study is to evaluate the potential prognostic and diagnostic value of MCT1, MCT4 and CD147 gene expression in the blood of patients with PCa at diagnosis and throughout the treatment.
Materials and Methods
Patients
In this study, prostate cancer patients from the oncology care laboratory of the ABC Medical School and healthy male donors (control group) were included. Inclusion criteria were men older than 18-year-old with prostate cancer confirmed by anatomopathological exam with no previous oncological treatment. Men with other prostate alteration than neoplasia and/or with comorbidities mainly diabetes mellitus were excluded from this study. The patients underwent three to five serial peripheral blood collections: at diagnosis (baseline), three months, six months, nine months and 12 months after initiating chemotherapy treatment. Healthy donors (control) underwent only one peripheral blood collection. Those men reported neither prostate disease nor other comorbidities. This study was approved by the Institutional Research Ethics Committee (protocol 024/2008, approved on April 23rd, 2008). All participants included read and signed an Informed Consent Form.
Total RNA extraction and cDNA synthesis
For gene expression analysis of the selected targets, 15 mL of peripheral blood were collected by venipuncture. Total RNA was isolated from the leukocyte fraction using the TRIzol reagent (TRIzol LS Reagent, Thermo Fisher Scientific, Waltham, MA, and USA), according to the manufacturer’s recommendations. The concentration and A260/A280 ratio of total RNA were measured by spectrophotometry with NanoDrop Lite (Thermo Fisher Scientific, Waltham, MA, USA).
cDNA was synthesized from 5μg of total RNA using the QuantiNova Reverse Transcription Kit (Qiagen, Hilden, Germany), according to the manufacturer’s recommendations.
Analysis of gene expression
Specific primers for the target genes were designed using Primer3 Input 0.4.0 software (available at http://frodo.wi.mit. edu/primer3/.) Primers sequences and their amplicons were: MCT1 for - TACCTCCAGACTCTCCTGGC and MCT1 Rev- GTCCCCTCCGCAAAGTCTAC (205 bp amplicon); MCT4 For- CGTTCTGGGATGGGACTGAC and MCT4 Rev- ATGTGCCTCTGGACCATGTG (216 bp amplicon); CD147 For- CCGTAGAAGACCTTGGCTCC and CD147 Rev- TACTCTCCCCACTGGTCGTC (169 bp amplicon); β-Actin For- CCCTGGAGAAGAGCTACGAG and β-Actin Rev- CAATGCCAGGGTACATGGTG (225 bp amplicon). Expression of the genes was evaluated by RT-qPCR in an Applied Biosystems 7500 Real Time PCR Systems thermocycler (Applied Biosystems, Foster City, CA, USA) with a final volume of 15μL, using SYBR Green 1X (SYBR Green dye-Quantitec SYBR Green PCR kit, Qiagen, Hilden, Germany), 0.25μM of each primer, with the following thermal conditions: one hot start step at 95°C for ten minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 25 seconds.
Target gene expression was normalized using the detected reference gene (β-Actin) expression. The difference in inter and intragroup relative gene expression was assessed by the 2-ΔCq. The results were presented as difference of expression followed by range (minimum and maximum) [28,29].
Statistical analysis
The results of gene expression analysis were expressed as mean ± standard deviation of the mean (SDM). To describe the quantitative variables (Shapiro-Wilk, p= 0.05) we used mean, standard deviation, minimum and maximum. The Mann-Whitney test was performed to analyze the association between the expressions of the target genes grouped into risk groups according to the Gleason score (low risk, intermediate risk, high risk and undetermined risk). To study the correlation between PSA values and clinical data with gene expression, the Spearman correlation test was performed. A Receiving Operating Characteristic (ROC) curve analysis was performed to evaluate the accuracy and establish a cut-off point for the markers. All analyzes assumed a 95% confidence level. The programs used were Stata version 11.0 and GraphPad Prism version 6 (GraphPad Software, CA, USA). The level of significance was set at 5% (descriptive value of p = 0.05).
Results
In this study, 27 prostate cancer and 26 healthy donors were included. The clinical characteristics of the 27 patients included in this study are described in Table 1. Patients were separated into groups according to the Gleason score and associated risk: Gleason score under seven (low risk), equal to seven (intermediate risk) and greater than seven (high risk). Three patients did not have the Gleason score determined. Risk stratification as determined by the Gleason score refers to the aggressiveness of the tumor and estimates the risk of recurrence or progression of the disease [30].
Table 1:Clinical characteristics of patients
Characteristics |
n |
% |
Gleason Score |
||
< 7 (low risk) |
9 |
33.33 |
= 7 (intermediate risk) |
11 |
40.74 |
> 7 (high risk) |
4 |
14.81 |
Indeterminate |
3 |
11.11 |
Treatment |
||
Radical prostatectomy |
18 |
66.66 |
Hormone therapy |
5 |
18.51 |
Bolla |
3 |
11.11 |
Indeterminate |
1 |
3.7 |
Surgical specimen risk |
|
|
Low risk |
4 |
14.81 |
Intermediate risk |
10 |
37.03 |
High risk |
3 |
11.11 |
Does not apply/indeterminate |
10 |
37.03 |
Margin |
|
|
Free |
11 |
40.74 |
Compromised |
5 |
18.51 |
Does not apply/indeterminate |
11 |
40.74 |
Postoperative Staging |
||
T2N0ouX |
10 |
37.03 |
T3aN0ouX |
4 |
14.81 |
T3bN0ouX |
3 |
11.11 |
Does not apply/indeterminate |
10 |
37.03 |
Presence of metastase |
|
|
Yes |
2 |
7.4 |
No |
25 |
92.6 |
Mean (sd) |
Minimum - Maximum |
|
Age |
66,4 (7,9) |
52.0 – 82.0 |
PSA |
33,5 (46,8) |
3.4 - 150 |
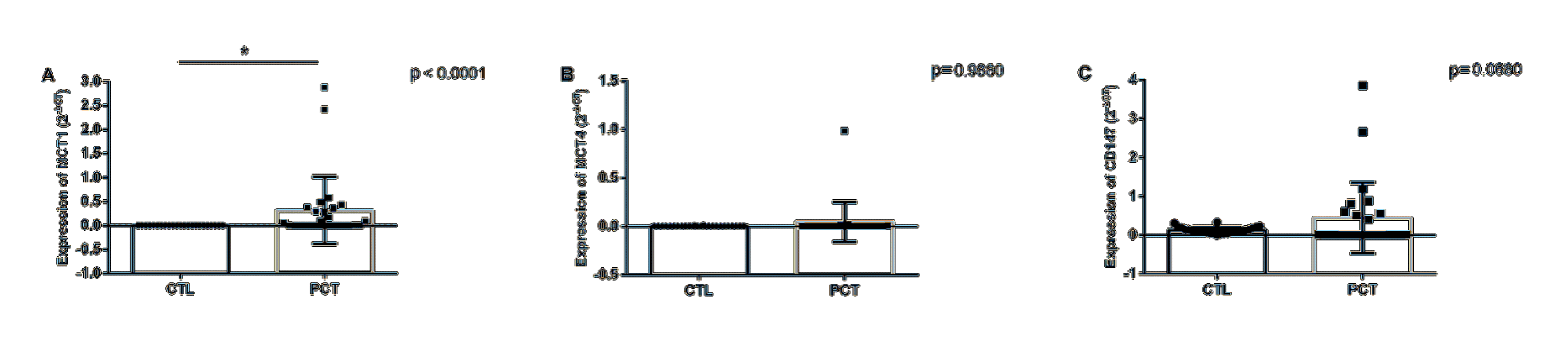
Expression of the three target genes could be detected in peripheral blood samples from both in patients and in healthy men (controls). Initially, a comparison between the expression profiles of these markers in patient and control samples was made (Figure 1, Table 2).
Table 2: Differential expression of the markers studied in patients with PCa at diagnosis and in healthy donors.
Gene |
Fold change |
p* |
MCT1 |
97 (19 – 477) |
<0.0001 |
MCT4 |
1.46 (0.28 – 7.6) |
0.988 |
CD147 |
3.52 (0.05-11.9) |
0.068 |
*Mann-Whitney; CI95%: confidence interval 95%. Fold change is expressed in mean + minimum and maximum
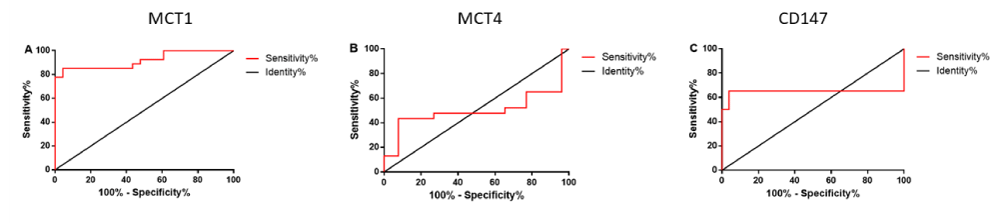
In order to estimate the predictive capacity and to establish cutoff points for the proposed markers for the diagnosis of PCa, an area analysis under the ROC curve was performed (Figure 2). The area under the ROC curve presented a value of 0.91 (95% CI = 0.83 - 0.99; p<0.0001) for MCT1; 0.50 (95% CI = 0.32- 0.68, p = 0.98) for MCT4 and 0.64 (95% CI = 0.46 - 0.83, p = 0.06) for CD147.
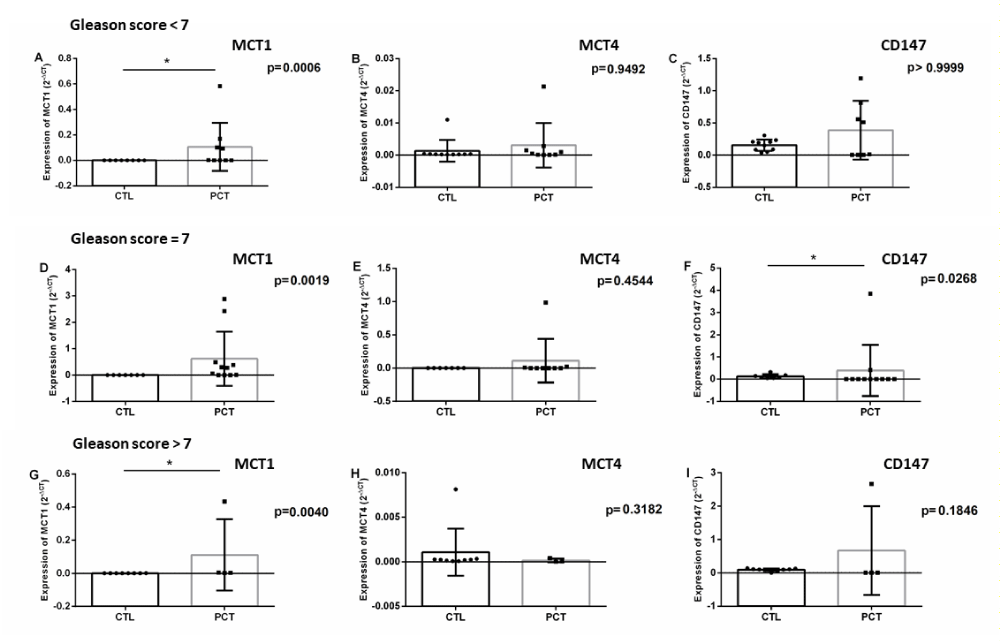
As previously stated, clinical and biopsy characteristics are used to calculate the Gleason score. This score is related to the prognosis, aggressiveness and staging of the disease. Thus, with the patients grouped according to their Gleason score, a comparison was made between the expression profiles of the markers in the blood of the patients at diagnosis between the three groups of patients (low, intermediate and high risk) and healthy donors (Table 3, Figure 3).
Figure 3: Differential expression of the proposed markers in patients’ blood according to Gleason score and controls (A - MCT1 of patients with Gleason score <7; B - MCT4 of patients with Gleason score <7; C - CD147 of patients with Gleason score <7; D - MCT1 of patients with Gleason score = 7; E - MCT4 of patients with Gleason score = 7; F - CD147 of patients with Gleason score = 7; G – MCT1 of patients with Gleason score > 7; H - MCT4 of patients with Gleason score > 7; I - of patients with Gleason score > 7).
Table 3: Differential expression of the markers studied between the blood of the patients with PCa separated into groups according to the Gleason score and healthy donors.
Gleason Score |
MCT1 |
p* |
MCT4 |
p* |
CD147 |
p* |
< 7 |
93.6 |
0.0006 |
2.02 |
0.9492 |
2.49 |
0.999 |
= 7 |
95.2 |
0.0019 |
1.63 |
0.4544 |
3.03 (0.007 – 4.17) |
0.0268 |
> 7 |
103.9 |
0.004 |
0.62 |
0.3182 |
7.19 (0.04 – 26.7) |
0.1846 |
* Mann-Whitney; 95% CI: 95% confidence interval. Difference in expression calculated by 2(-ΔCq) and expressed in fold change (mean + minimum/ maximum)
The difference in gene expression of patients forming a single group and grouped according to the Gleason score was also compared throughout the treatment and correlated with the gene expression of the donors with serum PSA level of the patients and other clinicalpathological data. No significant correlation was found between the expressions of markers in the peripheral blood of patients with these parameters. In order to verify the possibility of correlation between serum levels of total pre-treatment PSA and expression of the markers studied at diagnosis, the Spearman correlation test was performed. No correlation was found between the pre-treatment total PSA measurements and the genes under study (MCT1: r = 0.074, p = 0.7. MCT4: r = 0.17, p = 0.43. CD147: r = 0,19; p = 0.35).
Discussion
In this study, we aimed to evaluate the potential prognostic and diagnostic value of MCT1, MCT4 and CD147 gene expression in the blood of patients with PCa at diagnosis and throughout the treatment. The expression of these three genes could be detected in both PCa and healthy donors.
The MCT1, MCT4 and CD147 genes have basal expressions in leukocytes under physiological conditions, which explain their detection in the blood of healthy donors [31]. Increases in the expression of these genes in the blood of patients with prostate cancer can be explained by the presence of CTCs that, as is the case in tumor cells, demand a greater energy production [14,15]. As previously mentioned, the Warburg effect results in an increase in lactic acid production, which leads to increased expression of these genes [16,17].
MCT1 was most highly expressed gene in peripheral blood samples of patients at diagnosis (mean expression is 97 times higher than in healthy donors), which makes it a potential marker of diagnosis of PCa in liquid biopsies. CD147, on the other hand, has an increased expression with a tendency towards statistical significance. Future studies with increased sampling may help shed light on the value of this gene as a diagnostic marker for PCa in peripheral blood samples. Expression of MCT4 did not differ between patient and the control groups.
PSA is a kallikrein-related serine protease produced by both normal and malignant epithelial prostate cells. In this way, it is a prostate - but not prostate tumor -specific protein [5]. Thus, serum PSA levels increase for various causes such as prostatitis, benign prostatic hyperplasia, and tumor. Even so, the introduction of screening of serum PSA values as a marker of prostate cancer diagnosis (along with clinical data, rectal examination and Gleason score) is associated with an increase in disease detection and a substantial decline in prostate cancer mortality [5,6]. Elevated PSA levels are associated with an increased risk of developing CP and with a higher pathological grade and an increased risk of metastatic disease [6].
Serum PSA is predominantly associated with alpha-1- antiquimotrypsin. Unassociated PSA levels are known as free PSA (Total PSA - Complexed PSA) and are associated with greater specificity to detect PC in men with total PSA between 4 and 10 ng / mL [6]. Men with total PSA> 4ng / mL have indication for cancer screening, but most have no histological changes on biopsy; on the other hand, men below this cutoff may harbour tumor cells in their prostate [5]. In fact, considering the intermediate values of PSA (4.0 - 10 ng/mL), only 26% of the patients in this category have prostate cancer [32]. An accurate cutoff value of total PSA that can facilitate the detection of PC with high sensitivity and specificity in healthy men has not yet been defined [6]. Thus, patients who are within the limit range for malignancies would strongly benefit from the addition of new markers that aid in this classification. In order to better distinguish between benign and malignant prostate abnormalities in men over 50 years with normal digital rectal examination and PSA between 4-10 ng / mL and reduce unnecessary biopsies, the PHI (prostate health index) was stablished. This sorological test is a result of three PSA isoforms (proPSA [-2], free PSA and total PSA) analysis [6]. When the evaluation of the 3 PSA isoforms used in the PHI is added to the one of the peptide hK2 (kallikrein-related peptide 2) and clinical data of the patient, there is the 4K score [6]. According to the author, retrospective studies show that the score 4K was more accurate in clinically predicting a diagnosed PC and its aggressiveness than measures of PSA and age. A recent meta-analysis showed that the 4K score is associated with an 8-10% improvement in predicting biopsy-confirmed CP, indicating that its use could potentially eliminate the number of prostate biopsies currently performed by 48- 56%. However, the 4K score has not yet been approved by the FDA and there are no studies demonstrating the difference in accuracy between it and the PHI.
However, some studies have questioned the utility of PSA use in clinical routines and the overtreatment associated with overdiagnosis of PCa [33,34]. Modifications in the US and Canadian government recommendations which led to decreased use of PSA in the routine screening of PCa are associated with a diagnosis with a higher stage, data which evidences of the efficacy of its measures [35]. Even so, it is a method with decreased specificity and an increased incidence of false positives in patients with benign prostatic hyperplasia [36]. Considering the presented data, the evaluation of MCT1 expression in the peripheral blood of these patients could be a tool to aid in the diagnosis of PCa.
The ROC curve test is used to establish cut-off values for the diagnosis of disease, with the sensitivity (ability to detect disease) and specificity (ability to minimize false-positive results) for each value obtained for the individuals in this study. The area under the curve ranges from 0 to 1 and is related to the accuracy of the test in diagnosing the disease [37]. The MCT1 marker presented the highest area under the curve [0.91 (95% CI = 0.83 - 0.99; p <0.0001)], with the cut-off value of 2-ΔCq > 0.000589, with a sensitivity of 85.19% (95% CI = 66.27% to 95.81%) and a specificity of 95.65% (95% CI = 78.05% to 99.89%), values that confirm its potential as a blood marker of PCa. MCT4 and CD147 had low area under the curve [0.50 (95% CI = 0.32 - 0.68 p = 0.98) and 0.64 (95% CI = 0.46-0.83; p = 0.06), respectively]. MCT4 cannot be characterized as potential blood markers of PCa; however, CD147 AUC, although lower than that obtained for MCT1, is similar to that described for % free PSA (0,64) and higher than that described for free PSA (0,615) [38]. Future studies with increased sampling may help establish cut-off values for these genes and help establish them as diagnostic markers for PCa.
When evaluating the expression of the markers in the patients grouped according to their Gleason score, MCT1 was observed not only to present an increase of expression with statistical significance in the three groups (low, intermediate and high risk), as this increased expression accompanies the increase in the score and, consequently, the severity of the disease. Therefore, in addition to being a candidate for the diagnostic marker of PCa, MCT1 can be considered a marker of prognosis, since it is higher in patients with higher risk stratification.
In this work, we didn´t find any correlation between gene expression and clinical data. Different from our results, associations between MCT1, MCT4 and CD147 and clinical and pathological data of the patients, such as age and PSA have already been described [22]. However, this correlation was obtained between the expression of the markers in tumor tissue, and not in blood samples as evaluated here.
Proposed markers play an important role in maintaining the viability of cancer cells and an increase in their expression is related to an adaptation to the tumor microenvironment affected by the Warburg effect. For this reason, the MCT1 and MCT4 members of the monocarboxylate transporter family, along with their chaperone CD147 may be considered potential therapeutic targets for the treatment of cancer and need to be better characterized. Liquid biopsy is becoming increasingly important in the diagnosis and follow-up of diseases, since besides helping monitor established markers, it can
replace more invasive and often unnecessary surgical procedures, which put patients’ health at risk. The results of this study show that patients with PCa and healthy donors have differing expressions of MCT1, MCT4 and CD147 which can be detected in peripheral blood samples. MCT1 gene was the marker that presented the greatest statistically significant difference of expression between patients and donors, suggesting it is a potential diagnostic marker of PCa. This difference in expression directly accompanies the risk stratification proposed by the Gleason score, which also makes this gene a potential marker of prognosis. Together with PSA assessments, MCT1 expression may be useful for monitoring prostate cancer patients throughout and after their treatment, as well as their family members or other men at risk of developing the disease.
Acknowledgements
This work was supported by FAPESP grant 2017/ 03558-3.
References
- Instituto Nacional do Câncer (INCA). Estimativa 2018. Síntese de resultados e comentário.
- Adjakly M, Ngollo M, Dagdemir A, Judes G, Pajon A, Karsli-Ceppioglu S, et al. Prostate cancer: The main risk and protective factors-Epigenetic modifications. Ann Endocrinol. 2015; 76: 25-41.
- Mossanen M, Krasnow RE, Nguyen PL, Trinh QD, Preston M, Kibel AS. Approach to the patient with high-risk prostate cancer. Urol Clin North Am. 2017; 44: 635-645.
- Carlaw KR, Woo HH. Evaluation of the changing landscape of prostate cancer diagnosis and management from 2005 to 2016. Prostate Int. 2017; 5: 130-134.
- Eastham J. Prostate cancer screening. Investig Clin Urol. 2017; 58: 217-219.
- Hatakeyama S, Yoneyama T, Tobisawa Y, Ohyama C. Recent progress and perspectives on prostate cancer biomarkers. Int J Clin Oncol. 2017; 22: 214-221.
- Gordetsky J, Epstein J. Grading of prostatic adenocarcinoma: current state and prognostic implications. Diagn Pathol. 2016; 11: 25.
- Neto AS, Tobias-Machado M, Wroclawski ML, Fonseca FL, Pompeo AC, Del Giglio A. Molecular oncogenesis of prostate adenocarcinoma: role of the human epidermal growth factor receptor 2 (HER-2/neu). Tumori. 2010; 96: 645-649.
- Ma Y, Luk A, Young FP, Lynch D, Chua W, Balakrishnar B, et al. Droplet digital PCR based androgen receptor variant 7 (AR-V7) detection from prostate cancer patient blood biopsies. Int J Mol Sci. 2016; 17: 1264.
- Strati A, Markou A, Parisi C, Politaki E, Mavroudis D, Georgoulias V, et al. Gene expression profile of circulating tumor cells in breast cancer by RT-qPCR. BMC Cancer. 2011; 11: 422.
- Delgado PO, Alves BC, Gehrke FS, Kuniyoshi RK, Wroclavski ML, Del Giglio A, et al. Characterization of cell-free circulating DNA in plasma in patients with prostate cancer. Tumour Biol. 2013; 34: 983-986.
- Wroclawski ML, Serpa-Neto A, Fonseca FL, Castro-Neves-Neto O, Pompeo AS, Machado MT, et al. Cell-free plasma DNA as biochemical biomarker for the diagnosis and follow-up of prostate cancer patients. Tumour Biol. 2013; 34: 2921-2927.
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011; 144: 646-674.
- Warburg O, Wind F, Neglein E. Metabolism of tumors in the body. J Gen Physiol. 1927; 8: 519-530.
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324: 1029-1033.
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999; 343: 281-299.
- Izumi H, Torigoe T, Ishiguchi H, Uramoto H, Yoshida Y, Tanabe M, et al. Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treat Rev. 2003; 29: 541-549.
- Pérez-Escuredo J, Van Hée VF, Sboarina M, Falces J, Payen VL, Pellerin L, et al. Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta. 2016; 1863: 2481-2497.
- Pinheiro C, Penna V, Morais-Santos F, Abrahão-Machado LF, Ribeiro G, Curcelli EC, et al. Characterization of monocarboxylate transporters (MCTs) expression in soft tissue sarcomas: distinct prognostic impact of MCT1 sub-cellular localization. J Transl Med. 2014; 12: 118.
- Halestrap AP. The SLC16 gene family – structure, role and regulation in health and disease. Mol Aspects Med. 2013; 34: 337-349.
- Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, et al. Increased Expression of Monocarboxylate Transporters 1, 2 and 4 in Colorectal Carcinomas. Virchows Arch. 2008; 452: 139-146.
- Pértega-Gomes N, Vizcaíno JR, Miranda-Gonçalves V, Pinheiro C, Silva J, Pereira H, et al. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer. 2011; 11: 312.
- Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012; 64: 1-9.
- Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012; 18: 1319-1330.
- Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related Issues of CD147. Cancer Genomics Proteomics. 2010; 7: 157-169.
- Arendt BK, Walters DK, Wu X, Tschumper RC, Huddleston PM, Henderson KJ, et al. Increased expression of extracellular matrix metalloproteinase inducer (CD147) in multiple myeloma: role in regulation of Boyd myeloma cell proliferation. Leukemia. 2012; 26: 2286-2296.
- Luz MCB, Perez MM, Azzalis LA, Sousa LVA, Adami F, Fonseca FLA, et al. Evaluation of MCT1, MCT4 and CD147 genes in peripheral blood cells of breast cancer patients and their potential use as diagnostic and prognostic markers. Int J Mol Sci. 2017; 18: 170.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009; 55: 611-622.
- D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999; 17: 168-172.
- Merezhinskaya N, Ogunwuyi SA, Mullick FG, Fishbein WN. Presence and localization of three lactic acid transporters (MCT1, -2, and -4) in separated human granulocytes, lymphocytes, and monocytes. J Histochem Cytochem. 2004; 52: 1483-1493.
- Ross T, Ahmed K, Raison N, Challacombe B, Dasgupta P. Clarifying the PSA grey zone: The management of patients with a borderline PSA. Int J Clin Pract. 2016; 70: 950-959.
- Etzioni R, Gulati R, Tsodikov A, Wever EM, Penson DF, Heijnsdijk EA, et al. The prostate cancer conundrum revisited. Cancer. 2012; 118: 5955-5963.
- Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian Cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012; 104: 125-132.
- Zakaria AS, Dragomir A, Brimo F, Kassouf W, Tanguay S, Aprikian A. Changes in the outcome of prostate biopsies after preventive task force recommendation against prostate-specific antigen screening. BMC Urology. 2018; 18: 69.
- Filella X, Foj L. Prostate Cancer Detection and Prognosis: From Prostate Specific Antigen (PSA) to Exosomal Biomarkers. Int J Mol Sci. 2016; 17: 1784.
- Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013; 4: 627-635.
- Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multi-center study of [-2] Pro-Prostate-Speci_c Antigen (PSA) in combination with PSA and Free PSA for prostate cancer detection in the 2.0 to 10.0 ng/mL PSA range. J Urol. 2011; 185: 1650-1655.
Citation: Eythimios P, Sevasti M, Periklis T and Chrisostomos S. Thanatophoric Dwarfism Complicating Pregnancy - An Extremely Rare Case. Clin Oncol. 2019; 2(3): 1015.
PDF DownloadClinics Oncology © All Rights Reserved. 2018