ISSN: 2688-562X
Review Article
Role of Cyclooxygenase-2 – Peroxisome Proliferator-Activated Receptor Gamma Pathway in the Pathogenesis of Sebaceous Gland Carcinoma
Tanaya Bhattacharya1, Perumal Jayaraj1, Juhi Arora2, and Seema Sen3*
- 11Department of Zoology, University of Delhi, India
- 22Department of Biochemistry, University of Delhi, India
- 33Department of Ocular Pathology, All India Institute of Medical Sciences, India
- *Corresponding author: Seema Sen, Department of Ocular Pathology, Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi – 110029, India,
- Received: Jul 15, 2019 Accepted: Jul 26, 2019 Published: Jul 30, 2019
Abstract
Sebaceous gland carcinoma (SGC) is an uncommon malignancy of the skin, known to be very aggressive, recurrent and with a tendency to metastasize. Owing to a low global prevalence, there are significant lacunae in understanding the pathogenesis of SGC. In this review, the role of Cyclooxygenase-2-Peroxisome proliferator-activated receptor gamma signaling pathway in SGC including that of the eyelids has been discussed. Findings of a moderate to high expression of Cyclooxygenase-2 (COX-2) activity in a few studies suggest its probable role as a marker for pathogenesis of SGC. Peroxisome proliferator-activated receptor (PPAR) subtype, PPARγ is also reported to show association with the level of differentiation in sebaceous gland tissues, especially in case of SGC. Also, PPARγ agonists are evidenced to show anti-tumorigenesis properties. In eyelid SGC, studies are suggestive of an overexpression of COX-2. Further, successively decreasing levels of PPARγ expression is shown to be associated with significant loss of differentiation. Though the evidence available is discussed, however, to better understand the role of COX-2 – PPARγ pathway in pathogenesis of SGC further research is warranted.
Keywords:
Cyclooxygenase-2 (COX-2); Sebaceous Gland Carcinoma (SGC); Peroxisome Proliferator-Activated Receptor Gamma (PPARγ); Eyelid
Introduction
Non-melanoma skin cancers are the predominant variety of cancers of the periocular region, primarily including basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and sebaceous gland carcinoma (SGC) [1]. Being the more preponderant varieties, both BCC and SCC are extensively studied, whereas, the etiology of SGC is least explored except for evidence of its high prevalence in the eyelids [2]. At the molecular level, apart from the involvement of p53 tumor suppressor gene, Wnt, c-myc and hedgehog signaling pathways [3,4], not much is known regarding pathogenesis of SGC.
Extensive research for identification of molecular markers indicates over expression of proteins related to angiogenesis, inflammation and cell proliferation in SGCs.Cyclooxygenase-2 (COX-2) is one such protein whose over expression may be associated with SGC [5,6]. It is a subtype of cyclooxygenases, which are enzymes involved in the metabolism of fatty acids and primarily conversion of arachidonic acid to prostaglandin. With the extensive study of the role of COX-2 in tumor development and progression, elevated expressions of this enzyme are reported in several types of cancer [7-9].
Cell proliferation of sebaceous gland cells leads to an increase in lipogenesis and thus sebum formation, one of the proteins responsible for which is Peroxisome Proliferator Activated Receptor Gamma (PPARγ) [10]. It belongs to the nuclear hormone receptor family that is known to modulate the function of adipose tissues, inflammation and regulation of cell cycle. PPARs are also known to regulate the growth of cancer cells [11,12].
The COX-2 – PPARγ signaling pathway is therefore suggested to be playing a significant role in the pathogenesis of SGC.
Sebaceous Glands and its Carcinoma
Sebaceous glands: Development, function and regulation
Excluding the palms and soles, sebaceous glands are ubiquitous in areas of the skin having hair follicles. With preponderance on the face and scalp, these secrete a waxy, lipid-rich fluid called sebum that nourishes the hair follicle [13]. Sebaceous glands are composed of three distinct zones: the peripheral, the maturation and the necrotic zones. Cells of the peripheral zone constantly undergo mitosis and move towards the center of the gland while differentiating. Cells of the maturation zone are called sebocytes, store lipids in their cytoplasm and thereby gradually increase in size. Once fully grown, they move towards the necrosis zone where they are fragmented and the lipid content is released as sebum [4,13,14].
The major role of sebum is to act as a barrier on the surface of the skin, defending it against pathogens and protecting it from dehydration. Cholesterol, cholesteryl esters, triacylglycerides, diacylglycerides and other fatty acids are the main components of human sebum [4]. The type of sebum produced in the eyelids is called meibum as it is secreted by the meibomian glands. Meibum averts the sticking of lower and upper eyelids with each other by providing lubrication and delays the evaporation of tears [15].
A variety of hormones, cytokines, signaling molecules and other factors involved in lipid metabolism maintain the homeostasis of the sebaceous glands. Signals sent to its progenitor cells bring about the renewal of these glands thereby controlling the balance between their proliferation and differentiation [13]. An imbalance leads to various pathologies of the sebaceous gland.
The sebaceous glands play a reasonably important role in our body. Origin of several skin disorders including SGC has been directly linked to the down-regulation of skin homeostasis and imbalance in the reparative process of sebaceous glands [14]. Three pathways are known to play a role in the development and functional regulation of sebaceous glands, namely, the Wnt, c-myc and the Hedgehog signaling pathways [4]. An imbalance in any of these pathways may be responsible for tumorigenesis in the sebaceous glands (Table 1). Also, a significant nuclear over expression of p53 is observed in periocular SGC tissue [16]. Studies also suggest that mutational inactivation of p53 may be involved in the development of SGC [17].
Table 1: Signalling Pathways associated with SGC
Pathway |
Abnormal Expression |
Association with SGC |
References |
Wnt/β-catenin |
Overexpression of β-catenin |
Initiation of sebaceous tumors, metastasis |
[85–87] |
C-myc |
Overexpression of c-myc |
Hyperplasia of sebaceous glands |
[88,89] |
Hedgehog |
Ectopic hedgehog expression (due to gain-of-function mutation in its receptor) |
Hyperplasia of sebaceous glands, metastasis, Overexpression of c-myc |
[87,90,91] |
p53 |
Expression of mutant form of p53 |
Neoplasia of sebaceous glands |
[16,17] |
Sebaceous gland carcinoma: Distribution, diagnosis and therapy
SGC accounts for upto 4.6% of all malignant skin tumors and can be broadly classified into peri-ocular/ocular and extra-ocular, with the former having an incidence of nearly 75% [18-20]. Its similarity in appearance with benign lesion/nodules and difficulty in identifying poorly differentiated cells during histopathological analyses are the main reasons for its frequent misdiagnosis. This leads to its diagnosis at an advanced stage of the disease and therefore delayed treatment.
As per the current guidelines, the mainstay of treatment of ocular SGC that is diagnosed early is wide surgical resection under frozen section or Moh’s micrographic surgery control followed by eyelid reconstruction. An emerging technique of posterior lamellar resection of the eyelids with reconstruction, holds the promise of decrease in of its recurrence [21,22]. Recently through a retrospective analysis, Takagawa et al., suggested radiotherapy as an alternative to surgery for provided the tumor is localized and is less than 10mm in size [23].
In late cases - orbital exenteration, cryotherapy, radiation therapy, chemotherapy, or combination therapy are used [21]. Chemotherapy with carboplatin and pembrolizumab after orbital exenteration has been reported to be an effective treatment in a recent case of recurrent and metastatic SGC [24]. Neoadjuvant chemotherapy combining 5-Fluorouracil with carboplatin was also found to be useful in management of SGC [25]. Further, in late cases, multimodal therapy has been shown to improve both visual prognosis and survival [21,26].
Role of COX-2 – PPARγ PATHWAY in the Development of SGC
The probable role of COX-2 and PPARγ in the pathogenesis of SGC is reviewed in the following sections.
Role of COX-2 in cancer and SGC
Cyclooxygenases are known to be present as two isoforms: COX- 1 and COX-2. COX-1 is expressed in most tissues and controls physiological functions like maintenance of gastric mucosal lining and renal blood flow regulation by mediating prostaglandin synthesis [27]. COX-2, uncommon in normal tissues, is induced in response to growth factors; pro-inflammatory cytokines, mechanical tissue damage, and UV light [28]. Many recent studies report higher expressions of COX-2 mRNA in colorectal, lung, breast and prostate cancers, thereby suggesting a possible role of COX-2 in promoting tumor development [29].
Overexpression of COX-2 is evidenced to be associated with inhibition of apoptosis, angiogenesis andmetastasis. This enzyme also shows peroxidase activity that can result in the conversion of procarcinogens to carcinogens. COX-2 has the potential to co-oxidize xenobiotics like benzopyrene to mutagens by its peroxidase activity in tissues like colon [30]. Also, reactive metabolites formed due to catalysis of arachidonic acid by COX-2, reportedly have carcinogenic properties [11,31]. Additionally, chronic inflammation that is a risk factor for carcinomas is associated with increased synthesis of prostaglandins induced by overexpression of COX-2 [10].
In a study on SGC tissue samples of human head and neck regions, Erovic et al., showed all tissues to be positive for COX-2 with 82% showing COX-2 overexpression [5]. Meyer et al. investigating COX-2 expression in skin tumors using tissue microarray technique, showed 86.9% of sebaceous adenomas to be COX-2 positive [12]. This indicates that COX-2 might have a significant role in the pathogenesis of sebaceous gland neoplasms.
At the molecular level, transgenic and/or knockout technologies are believed to have established the link between COX-2 and tumorigenesis [32]. Tachibana et al., report overexpression of COX-2 gene in mammary glands in a study using transgenic mice which, in multiparous females led to the formation of tumors with local and distant metastasis [11].
When treated with 7,12-dimethyl-benz[α]anthracene (DMBA, a carcinogen), transgenic mice are seen to develop several sebaceous gland adenomas of the skin. When studied further, prominent COX-2 overexpression and high prostaglandin production in basal sebocytes are observed in them [8], suggesting a probable association between the genesis of sebaceous adenoma and increased sebum production.
Another reason behind COX-2 being involved in tumor development could be attributed to its role in angiogenesis. Tsuji et al. report the pro-angiogenic effects of COX-2 overexpression in colon cancer cells, when inhibited by a selective COX-2 inhibitor - NS-398 [33]. It is a well-established fact that angiogenesis is one of the main features of cancer cells as new blood vessels provide nutrients to the growing tumor. An experimental model designed to include COX-2 overexpressing Caco-2 cells (transfected) and HCA-7 (Human colon adenocarcinoma cell line) cells expressing COX-2 constitutively, show high levels of angiogenic factors [34]. In colon cancer, overexpression of COX-2 promotes the formation of capillary-like networks by increasing the production of vascular endothelial growth factors [33].
From the aforementioned studies, it can be assumed that the level of COX-2 expression has a role in various cancers. Nevertheless, more studies are required to ascertain the exact association of COX-2 with SGC.
Role of PPARγ in cancer and SGC
PPARs belonging to the super family of nuclear hormone receptors are transcription factors that regulate the expression of genes in a ligand dependent manner. They function by forming heterodimers with specific ligands and retinoid X receptors (RXR), binding to specific DNA elements called PPAR response elements (PPRE). PPARs are frequently linked to regulation of cell differentiation, development, metabolism and tumorigenesis in higher organisms. A significant correlation of PPARs with various types of cancers such as lung, liver, colon, breast and skin cancers is well established [35,36].
The three subtypes of PPARs - PPARα, PPARδ and PPARγ show varied tissue distribution and different functions [36]. It is the involvement of PPARs in adipogenesis and lipid metabolism that links them to sebum production in the sebaceous glands. Rosenfield et al., in their study on rat preputial cells (that serve as a model for human sebocytes), report the presence of PPARδ and PPARγ mRNAs using RNase protection assay. Further, they report the accumulation of lipid droplets because of induction by ligands of PPARα and PPARγ [37]. This suggests a possible role of PPARs in regulating sebum production in human sebocytes.
Gene expression analyses show PPARα to be one of the main regulators of genes involved in lipid metabolism [38]. Devchand et al., in a study done on transgenic mice, show the deficiency of PPARα results in prolonged inflammatory response to lipid mediators, indicating thatPPARα has a physiological role in suppressing inflammation [39]. PPARα is expressed in cultured sebocytes and in human sebaceous glands where it increases the synthesis of antiinflammatory cytokines [40]. PPARα is also implicated for hepatocarcinogenesis in mice; however, there is no evidence of a similar effect in humans [41].
The role of PPARδ (also known as PPARβ) in cancer is, however, unclear. Few studies report its tumor promoting effect in many cancers while, others state that administration of its agonists suppresses cancer development [42]. Though PPARδ is known to play a significant role in lipid accumulation and terminal differentiation in keratinocytes, there is no evidence of its association with SGC [43].
The role of PPARγ as an anticancer agent is suggested in various studies as PPARγ agonists play a substantial role in promoting apoptosis, inducing terminal differentiation and impeding cell proliferation [42]. Arachidonic acid metabolites, 15d-PGJ2 and PGD2 act as the natural ligands for PPARγ [36]. Several experiments using pancreatic cancer cells reported PPARγ expression and these when treated with its ligands/agonists such as thiazolidinediones/3,3- diindolylmethane, resulted in apoptosis via endoplasmic stress cell response [44].
PPARγ expression facilitates sebocyte differentiation and lipid synthesis in sebaceous glands. Studies in mice lacking PPARγ in the skin cells demonstrate that development of sebaceous glands and adipose tissues require a functional PPARγ [37]. Keratinocytes of mice lacking PPARγ are shown to have increased epithelial tumor progression [45].
A recent study reports the definitive role of PPARγ in regulation of lipogenesis and cell proliferation in SZ95 sebocytes [46]. Dozsa et al., suggest that PPARγ is differentially expressed in human sebocytes that were normal as compared to those that were diseased. They emphasize that the level of expression of PPARγ is significantly lower in case of sebaceous adenomas and SGC as compared to in normal sebaceous gland tissue [47].
Role of COX-2 – PPARγ pathway
Prostaglandins that are produced by the action of cyclooxygenases on arachidonic acid also act as endogenous ligands of PPARs [48]. Further, arachidonic acid itself is a PPARγ ligand [49]. Interestingly, non-steroidal anti-inflammatory drugs (NSAIDs) such as thiazolidine derivatives are not only inhibitors of COX-2 but are also observed to act as ligands of PPARγ [50].
The archetypical inhibitor of COX-2, NSAIDs are known to reduce the risk of many cancers thus making COX-2 a major clinical target for anticancer therapy [51]. Selective COX-2 inhibitors like celecoxib and rofecoxib are proven to suppress and prevent tumorigenesis [52]. The growth of tumors is typically associated with immune suppression. It is believed that selective inhibition of COX-2 can restore the balance between interleukins 10 and 12 thereby promoting anti-tumor activity [53]. Further, treatment by NSAID sulindacsulfide reversed the apoptotic resistance shown initially by rat intestinal epithelial cells engineered to overexpress COX-2 [54]. Moreover, inhibition of cyclooxygenases by NSAIDs is said to result in an altered pathway for the metabolism of arachidonic acid. This is shown to decrease the number and size of intestinal polyps in case of colon cancers and suppression of skin related tumor developments [31]. Selective COX- 2 inhibitors are observed to be effective in treating colorectal cancers that evolve from adenomas [55]. In SZ95 (immortalized human sebaceous gland cell line) sebocytes, differentiation and lipid synthesis is regulated by a PPARγ/COX-2-mediated signaling pathway [56].
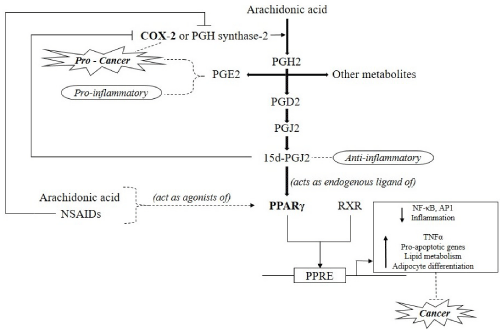
All of these are suggestive of the presence of a COX-2 – PPARγ signaling pathway (Figure 1). The conversion of the fatty acid - arachidonic acid to prostaglandin PGH2 is catalyzed by COX-2. Many enzymes catalyze PGH2 to produce prostaglandins such as PGE2 and PGD2 among others. Overexpression of both PGE2 and COX-2 are reported to promote carcinogenesis. Prostaglandin PGE2 is also pro-inflammatory in nature. Conversely, PGD2 is converted to 15d-PGJ2 that has been shown to have anti-inflammatory properties. Interestingly, 15d-PGJ2 has been observed to inhibit COX-2. The 15d-PGJ2 acts as an endogenous ligand of PPARγ that is a transcription factor involved in the activation of genes that suppress cancer development [9,57,58]. Moreover, it has been proposed in a study that COX-2 expression might be significantly controlled by a negative feedback loop which is regulated by PPARγ [59].
Figure 1: COX-2 – PPARγ Signalling Pathway- The conversion of the fatty acid - arachidonic acid to prostaglandin PGH2 is catalysed by COX- 2. Enzymes catalyse PGH2 to produce other prostaglandins (PGE2 and PGD2). PGD2 is converted to 15d-PGJ2 which acts as a ligand of PPARγ (9,57,59)
Abbreviations: PGH2 – Prostaglandin H2, PGE2 – Prostaglandin E2, PGD2 – Prostaglandin D2, PGJ2 – Prostaglandin J2, 15d-PGJ2 –15-deoxy- Δ12,14-PGJ2, NSAIDs – Non steroidal anti-inflammatory drugs, RXR – Retinoid X Receptor, PPRE - Peroxisome proliferator response element, NF-κB –Nuclear factor kappa-light-chain-enhancer of activated B cells, AP1
The reported involvement of this pathway in various types of cancers including, but not limited to, that of the breast, prostate gland, pancreas and lung is indicative of its association with cancer development [9,60,61]. Few studies suggest that COX-2 and its signaling pathway are strongly linked with non-melanoma skin carcinogenesis [62].
SGC of the Eyelids
Distribution
Among non-melanoma cancers of the eyelid, BCC is the most common type accounting for 80-95% of eyelid cancers and originates mostly from the lower eyelid or the medial canthus [1,63]. This is followed by SCC that accounts for about 9.2% and usually manifests as a nodular or ulcerative lesion. Periocular SCC commonly arises from the lower eyelid, upper eyelid or canthi [1,64]. It may in even originate from the bulbar or palpebral conjunctiva [65]. SGC accounts for only 1-5% of eyelid malignancies, but is the most aggressive amongst them [1]. Clinico-pathological studies based on the Indian subpopulation, however, suggest that BCC and SGC are more prevalent (upto 48% and 31% respectively), while SCC is the rarer variety (13-22%) of eyelid cancer [66,67].
The most common location of BCC is the face. SCC can occur in other areas and are often caused by constant and prolonged local irritation [68,69]. BCC and SCC affect particularly areas of the skin that are exposed to sunlight, especially ultraviolet (UV) radiation [69]. In contrast, there is little effect of UV exposure on the etiology of SGC [70]. However, a recent study has reported mutations caused by UV damage in 32 SGC cases by whole-exome sequencing and has found similarities with SCC [71].
Pathogenesis and diagnosis
Periocular SGC tumors can arise from the tarsal meibomian gland, the Zeis gland of the hair follicles or in rare cases from the sebaceous glands of the lacrimal caruncle [18,72,73]. These are highly aggressive tumors found predominantly in older women and tend to invade the regional lymph nodes as well as metastasize to various body parts including the brain [74]. In 50-60% cases, however, the cell of origin remains unknown [2].
Histopathological analysis reveals that well-differentiated tumor tissue appears as lobular masses with foamy, vacuolated cytoplasm that sometimes show central necrosis [26]. Poorly differentiated SGC tissue appears to have cells with pleomorphic nuclei and ‘appliqué pattern’ or peripheral cell necrosis [75]. Further, it reportedly shows pagetoid spread, a distinctive superficial pattern of ‘upward’ invasive spread of cancerous cells which is more prominent in the upper eyelids due to the abundance of meibomian glands [22,76].
Poorly differentiated eyelid SGC are frequently incorrectly diagnosed as SCC or BCC [21,22,77]. In its early stages, the SGC tumors mimic benign conditions like blepharoconjunctivitis or chalazion [78]. This often leads to misdiagnosis and a delay in treatment resulting in widespread local and distant metastases [2]. Recently, a study has reported ‘polymorphous vessels with yellow backgrounds’ as a characteristic feature of periocular SGC using dermoscopy [19].
Initially, SGC may develop as a painless chalazion-like nodule, an ulcerative lesion or as a thickening/inflammation of the eyelid akin to blepharitis [79,80]. It can subsequently spread to the conjunctiva and masquerade as conjunctivitis. Progressive loss of eyelashes has also been reported in a SGC case that was earlier misdiagnosed to be blepharoconjunctivitis [78]. As both COX-2 and PPARγ play a prominent role in inflammation, a feature commonly seen in earlier stages of SGC, it is possible that they may be involved in the development of SGC of the eyelids. Further, it has been reported that SGC nodules when visualized closely, appear yellowish because of their high lipid content [19,76]. This may be another reason to explore the role of PPARγ in SGC due to its direct involvement in lipogenesis.
COX-2 and PPARγ in eyelid SGC
Patel et al., from Canada, reported COX-2 overexpression in non-neoplastic sebaceous glands surrounding the tumor tissue in about half of the eyelid SGC tissue samples. The authors suggest that the expression of COX-2 may be an early indicator of neoplastic transformation and the aggressiveness of SGC [34]. Jayaraj et al., in a study based on the Indian subpopulation, report strong cytoplasmic overexpression of COX-2 in 80.6% eyelid SGC tissues (including both well and poorly differentiated SGC tissues). Further, they report a substantial association of COX-2 overexpression with reduced disease-free survival and suggest that COX-2 overexpression could be a prognostic predictor of aggressiveness in SGC [81]. These studies indicate that irrespective of the geographical distribution, COX-2 is overexpressed in SGC.
Overexpression of COX-2 may also be partially responsible for the loss of eyelashes and alopecia. Müller-Decker et al., in a study on transgenic mice, show COX-2 overexpression leads to sebaceous gland hyperplasia and consequent excessive sebum production in addition to delayed emergence of hair shaft and reduced density of hair follicle [82].
A few studies have reported that PPARγ is required by sebaceous glands for their proper differentiation [13,83,84]. In their study on eyelid SGC cases, Jayaraj et al. report negligible PPARγ expression in poorly differentiated SGC in contrast to PPARγ overexpression in 90.4% of well-differentiated SGC cases. Its high expression is also observed in cases of eyelid sebaceous adenoma and hyperplasia; which are suggestive of PPARγ being a possible diagnostic marker of well-differentiated SGC as the level of expression of PPARγ reduces with the loss of differentiation in the SGC tissue [81].
Though presently evidence of the possible association between COX-2 – PPARγ signaling pathway and pathogenesis of SGC especially that of the eyelids is not very strong, this possibility is worth exploring. Further, significant overexpression of COX-2 and progressive decline in PPARγ expression has been reported suggesting the presence of an inverse correlation between COX-2 and PPARγ in eyelid SGC.
Conclusion
There is substantial evidence of a functioning prostaglandin pathway in sebaceous glands. Prostaglandins are important for lipogenesis and adipogenesis in these glands and they are known to play a role in tumorigenesis. As COX-2 is involved in generation of prostaglandins, it is indicative that COX-2 plays a significant role in generation of benign tumors as well as in carcinogenesis. Several studies indicate an overexpression of COX-2 in SGC; nevertheless, more conclusive evidence is necessary to ascertain its role in pathogenesis of SGC. The role of PPARγ in lipogenesis and sebocyte differentiation is well established. However, only a few studies emphasize its association with SGC.
In eyelid SGC, over expression of COX-2 and reduction in PPARγ with loss of tumor differentiation is indicated. Therefore, COX-2 PPARγ pathway is possibly another molecular pathway in the pathogenesis of eyelid SGC. However, further studies are necessary to validate this theory.
References
- Lober CW, Fenske NA. Basal cell, squamous cell, and sebaceous gland carcinomas of the periorbital region. J Am Acad Dermatol. 1991; 25: 685-690.
- Wali UK, Al-Mujaini A. Sebaceous gland carcinoma of the eyelid. Oman J Ophthalmol. 2010; 3: 117-121.
- Joerger AC, Fersht AR. The p53 Pathway: Origins, Inactivation in Cancer, and Emerging Therapeutic Approaches. Annu Rev Biochem. 2016; 85: 375-404.
- Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008; 49: 271-281.
- Erovic BM, Al Habeeb A, Harris L, Goldstein DP, Kim D, Ghazarian D, et al. Identification of novel target proteins in sebaceous gland carcinoma. Head Neck. 2013; 35: 642-648.
- Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990; 76: 655-663.
- Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, et al. Cyclooxygenase-2 Expression Is Up-Regulated in Squamous Cell Carcinoma of the Head and Neck. Cancer Res. 1999; 59: 991-994.
- Müller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Fürstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci. 2002; 99: 12483-12488.
- Larsson K, Kock A, Idborg H, ArsenianHenriksson M, Martinsson T, Johnsen JI, et al. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc Natl Acad Sci. 2015; 112: 8070-8075.
- Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol Baltim Md. 1997; 11: 779-791.
- Tachibana K, Yamasaki D, Ishimoto K, Doi T. The Role of PPARs in Cancer. PPAR Res. 2008; 2008: 102737.
- Meyer S, Vogt T, Landthaler M, Berand A, Reichle A, Bataille F, et al. Cyclooxygenase 2 (COX2) and Peroxisome Proliferator-Activated Receptor Gamma (PPARG) Are Stage-Dependent Prognostic Markers of Malignant Melanoma. PPAR Res. 2009; 2009: 848645.
- Niemann C. Differentiation of the sebaceous gland. Dermatoendocrinol. 2009; 1: 64-67.
- Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol. 2012; 23: 928-936.
- Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961; 1: 39-45.
- Shalin SC, Sakharpe A, Lyle S, Lev D, Calonje E, Lazar AJ. P53 Staining Correlates with Tumor Type and Location in Sebaceous Neoplasms. Am J Dermatopathol. 2012; 34: 129-138.
- Gonzalez-Fernandez F, Kaltreider SA, Patnaik BD, Retief JD, Bao Y, Newman S, et al. Sebaceous carcinoma. Tumor progression through mutational inactivation of p53. Ophthalmology. 1998; 105: 497-506.
- Rao NA, Hidayat AA, McLean IW, Zimmerman LE. Sebaceous carcinomas of the ocular adnexa: A clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982; 13: 113-122.
- Satomura H, Ogata D, Arai E, Tsuchida T. Dermoscopic features of ocular and extraocular sebaceous carcinomas. J Dermatol. 2017; 44: 1313-1336.
- Urban FH, Winkelmann RK. Sebaceous Malignancy. Arch Dermatol. 1961; 84: 63-72.
- Knackstedt T, Samie FH. Sebaceous Carcinoma: A Review of the Scientific Literature. Curr Treat Options Oncol. 2017; 18: 47.
- Cicinelli MV, Kaliki S. Ocular sebaceous gland carcinoma: an update of the literature. IntOphthalmol. 2019; 39: 1187-1197.
- Takagawa Y, Tamaki W, Suzuki S, Inaba K, Murakami N, Takahashi K, et al. Radiotherapy for localized sebaceous carcinoma of the eyelid: a retrospective analysis of 83 patients. J Radiat Res. 2019: 4.
- Kodali S, Tipirneni E, Gibson PC, Cook D, Verschraegen C, Lane KA. Carboplatin and PembrolizumabChemoimmunotherapy Achieves Remission in Recurrent, Metastatic Sebaceous Carcinoma. Ophthal Plast Reconstr Surg. 2018; 34: e149-151.
- Kaliki S, Ayyar A, Nair AG, Mishra DK, Reddy VAP, Naik MN. Neoadjuvant Systemic Chemotherapy in the Management of Extensive Eyelid Sebaceous Gland Carcinoma: A study of 10 Cases. Ophthal Plast Reconstr Surg. 2016; 32: 35-39.
- Shields JA, Demirci H, Marr BP, Eagle RC, Shields CL. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004; 111: 2151-2157.
- Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, et al. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996; 56: 4424-4429.
- Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, et al. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001; 2: 544-551.
- Bol DK, Rowley RB, Ho C-P, Pilz B, Dell J, Swerdel M, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002; 62: 2516-2521.
- Eling TE, Thompson DC, Foureman GL, Curtis JF, Hughes MF. Prostaglandin H synthase and xenobiotic oxidation. Annu Rev PharmacolToxicol. 1990; 30: 1-45.
- Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep PR. 2010; 62: 233-244.
- Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001; 276: 18563-18569.
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998; 93: 705-716.
- Patel VR, Al-Khalifa S, Caissie AL, Callejo SA, Arthurs B, Burnier MN. Cyclooxygenase 2 (COX-2) Expression in Sebaceous Cell Carcinoma of the Eyelid. Invest Ophthalmol Vis Sci. 2002; 43: 3034.
- Zouboulis CC. Sebaceous glands and the prostaglandin pathway--key stones of an exciting mosaic. J Invest Dermatol. 2005; 125: x–xi.
- Michalik L, Wahli W. PPARs Mediate Lipid Signaling in Inflammation and Cancer. PPAR Res. 2008; 2008: 134059.
- Rosenfield RL, Kentsis A, Deplewski D, Ciletti N. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. J Invest Dermatol. 1999; 112: 226-232.
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990; 347: 645-650.
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996; 384: 39-43.
- Zouboulis CC. Sebaceous gland receptors. Dermatoendocrinol. 2009; 1: 77-80.
- Reddy JK, Azarnoff DL, Hignite CE. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980; 283: 397-398.
- Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012; 12: 181-195.
- Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004; 122: 971-983.
- Eibl G. The Role of PPAR-gamma and Its Interaction with COX-2 in Pancreatic Cancer. PPAR Res. 2008; 2008: 326915.
- Sertznig P, Reichrath J. Peroxisome proliferator-activated receptors (PPARs) in dermatology: Challenge and promise. Dermatoendocrinol. 2011; 3: 130-135.
- Mastrofrancesco A, Ottaviani M, Cardinali G, Flori E, Briganti S, Ludovici M, et al. Pharmacological PPARγ modulation regulates sebogenesis and inflammation in SZ95 human sebocytes. BiochemPharmacol. 2017; 138: 96-106.
- Dozsa A, Dezso B, Toth BI, Bacsi A, Poliska S, Camera E, et al. PPARγ-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytes. J Invest Dermatol. 2014; 134: 910-920.
- Spencer AG, Woods JW, Arakawa T, Singer II, Smith WL. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J Biol Chem. 1998; 273: 9886-9893.
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl AcadSci U S A. 1997; 94: 4318–4323.
- Zhang S, Gu H, Hu N. Role of Peroxisome Proliferator-Activated Receptor γ in Ocular Diseases. J Ophthalmol. 2015; 2015: 275435.
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002; 94: 252-266.
- Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998; 58: 409-412.
- Kambayashi T, Alexander HR, Fong M, Strassmann G. Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol. 1995; 154: 3383–3390.
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995; 83: 493–501.
- Steinbach G, Lynch PM, Phillips RKS, Wallace MH, Hawk E, Gordon GB, et al. The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. N Engl J Med. 2000; 342: 1946–1952.
- Zhang Q, Seltmann H, Zouboulis CC, Konger RL. Involvement of PPARgamma in oxidative stress-mediated prostaglandin E(2) production in SZ95 human sebaceous gland cells. J Invest Dermatol. 2006; 126: 42-48.
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995; 83: 813-819.
- Scher JU, Pillinger MH. 15d-PGJ2: The anti-inflammatory prostaglandin? Clin Immunol. 2005; 114: 100-109.
- Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000; 275: 28028–28032.
- KNOPFOVÁ L, ŠMARDA J. The use of Cox-2 and PPARγsignaling in anti-cancer therapies. ExpTher Med. 2010; 1: 257-264.
- Ravi Kiran Ammu VVV, Garikapati KK, Krishnamurthy PT, Chintamaneni PK, Pindiprolu SKSS. Possible role of PPAR-γ and COX-2 receptor modulators in the treatment of Non-Small Cell lung carcinoma. Med Hypotheses. 2019; 124: 98-100.
- Müller-Decker K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011; 30: 343-361.
- Cook BE, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999; 106: 746-750.
- Reifler DM, Hornblass A. Squamous cell carcinoma of the eyelid. Surv Ophthalmol. 1986; 30: 349-365.
- Tunc M, Char DH, Crawford B, Miller T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. Br J Ophthalmol. 1999; 83: 98-103.
- Abdi U, Tyagi N, Maheshwari V, Gogi R, Tyagi SP. Tumours of eyelid: a clinicopathologic study. J Indian Med Assoc. 1996; 94: 405-418.
- Kale SM, Patil SB, Khare N, Math M, Jain A, Jaiswal S. Clinicopathological analysis of eyelid malignancies - A review of 85 cases. Indian J Plast Surg. 2012; 45: 22-28.
- Khanolkar VR, Suryabai B. Cancer in relation to Usages. Three New Types in India. Arch Pathol. 1945; 40: 351-361.
- Rosso S, Zanetti R, Martinez C, Tormo MJ, Schraub S, Sancho-Garnier H, et al. The multicentre south European study “Helios”. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996; 73: 1447–1154.
- Hussain RM, Matthews JL, Dubovy SR, Thompson JM, Wang G. UV-independent p53 mutations in sebaceous carcinoma of the eyelid. Ophthal Plast Reconstr Surg. 2014; 30: 392–395.
- North JP, Golovato J, Vaske CJ, Sanborn JZ, Nguyen A, Wu W, et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat Commun. 2018; 9: 1894.
- Straatsma BR. Meibomian Gland Tumors. AMA Arch Ophthalmol. 1956; 56: 71-93.
- Shields JA, Shields CL. Sebaceous carcinoma of the glands of Zeis. Ophthal Plast Reconstr Surg. 1988; 4: 11-14.
- Raza Rizvi SA, Alam S, Akhtar K. Eyelid sebaceous gland carcinoma: Varied presentations and reconstruction outcome. Oman J Ophthalmol. 2018; 11: 21-27.
- Broekaert SMC, Flux K, Kyrpychova L, Kacerovska D, Ivan D, Schön MP, et al. Squared-Off Nuclei and “Appliqué” Pattern as a Histopathological Clue to Periocular Sebaceous Carcinoma: A Clinicopathological Study of 50 Neoplasms From 46 Patients. Am J Dermatopathol. 2017; 39: 275-278.
- Barsegian A, Shinder R. Eyelid Sebaceous Gland Carcinoma with Extensive Pagetoid Spread. Ophthalmology. 2017; 124: 858.
- Prieto-Granada C, Rodriguez-Waitkus P. Sebaceous Carcinoma of the Eyelid. Cancer Control. 2016; 23: 126-132.
- Margo CE, Lessner A, Stern GA. Intraepithelial sebaceous carcinoma of the conjunctiva and skin of the eyelid. Ophthalmology. 1992; 99: 227-231.
- Pereira PR, Odashiro AN, Rodrigues-Reyes AA, Correa ZMS, Filho JPDS, Burnier MN. Histopathological review of sebaceous carcinoma of the eyelid. J Cutan Pathol. 2005; 32: 496-501.
- Schmitz EJ, Herwig-Carl MC, Holz FG, Loeffler KU. Sebaceous gland carcinoma of the ocular adnexa - variability in clinical and histological appearance with analysis of immunohistochemical staining patterns. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 2277–2285.
- Jayaraj P, Sen S, Bhattacharya T, Arora J, Yadav S, Chhoker V, et al. Clinical relevance of cyclooxygenase 2 and peroxisome proliferator-activated receptor gamma in eyelid sebaceous gland carcinoma. Histopathology. 2016; 69: 268-275.
- Müller-Decker K, Leder C, Neumann M, Neufang G, Bayerl C, Schweizer J, et al. Expression of cyclooxygenase isozymes during morphogenesis and cycling of pelage hair follicles in mouse skin: precocious onset of the first catagen phase and alopecia upon cyclooxygenase-2 overexpression. J Invest Dermatol. 2003; 121: 661–668.
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPARγ Is Required for the Differentiation of Adipose Tissue In Vivo and In Vitro. Mol Cell. 1999; 4: 611–617.
- Furue M, Takemura M, Nishio K, Sato Y, Nagata S, Kan N, et al. Immunohistological Localization of Peroxisome Proliferator-Activated Receptor α and γ in Human Sebaceous Glands. Fukuoka Igaku Zasshi. 2016; 107: 199–203.
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434: 843–850.
- Niemann C, Unden AB, Lyle S, Zouboulis CC, Toftgård R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003; 100: 11873–11880.
- Kim N, Kim JE, Choung H-K, Lee MJ, Khwarg SI. Expression of Shh and Wntsignaling pathway proteins in eyelid sebaceous gland carcinoma: clinicopathologic study. Invest Ophthalmol Vis Sci. 2013; 54: 370–377.
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol CB. 2001; 11: 558–568.
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001; 28: 165–168.
- Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Exp Dermatol. 2006; 15: 667–677.
- Botchkarev VA, Fessing MY. Edar signaling in the control of hair follicle development. J Investig Dermatol Symp Proc. 2005; 10: 247–251.
Citation: Seema Sen, et al. Role of Cyclooxygenase-2 – Proliferator-Activated Receptor Gamma Pathway in the Pathogenesis of Sebaceous Gland Carcinoma. Clin Oncol. 2019; 2(3): 1014.
PDF DownloadClinics Oncology © All Rights Reserved. 2018